orbital diagram of lead
Combining waves can lead to constructive interference in which peaks line up with peaks or destructive interference. Orbital diagram of Astatine At 86.

Introduction To Molecular Orbital Theory
Lead Pb has an atomic mass of 82.

. To write the orbital diagram of leadPb you have to do the electron configuration of lead. Rhodium ionRh 3 electron configuration. The orbital diagram of lead shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons the 2p subshell has 6 electrons the 3s subshell has 2 electrons the.
Orbital diagram of Bismuth Bi 84. There are three rules followed for calculating the orbital diagram for an atom. The ground state electron configuration of rhodium is 1s 2 2s 2 2p 6 3s 2 3p 6.
1s is the closest and lowest energy orbital to the nucleus. Which means that it has 82 protons in its nucleus. Find out about its chemical and physical properties states energy electrons oxidation and more.
The order in which the orbitals are filled with electrons from lower energy to higher energy is. To write the orbital diagram of potassium K you have to do the electron configuration of potassium. Lead has an atomic number 82.
The electron configuration of manganese ion Mn 4 is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 3. 1s is the closest and lowest energy. Orbital diagrams Orbital box diagrams of all elements are mentioned in the.
The orbital diagram will be filled in the same order as described by the Aufbau principle. There are atomic orbitals in chemistry and the role of electronic configuration is that it tells us how many electrons are divided in their. Which has been discussed in detail above.
The relative energy levels of atomic and molecular orbitals are. Orbital diagram of Lead Pb 83. Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below.
This is clearly shown in the figure of the orbital diagram of rhodium. Orbital diagrams Orbital box diagrams of all. A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination.
Electronic configuration of the Lead atom in ascending order of orbital energies. 1s 2s. The orbital diagram of lead shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons the 2p subshell has 6 electrons the 3s subshell has 2 electrons the.
Aufbaus principle- This rule state that the lower energy orbital will be filled before the higher energy. Molecular Orbital Diagrams. As we that in a neutral element number of protons are equal to number of electrons.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s. 1s 2 2s 2 2p 6. 1134 gcm 3.
Orbital diagram of Polonium Po 85. From the above information we can say that manganese exhibits 2 3 and. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen.
Orbital diagram of Radon. Again Mn 4e Mn 4. Electron configurations have the format.
The first number is the principal quantum number n and the letter represents the value of l angular momentum. Which has been discussed in detail above.

Illustration Of The Orbital Interactions That Lead To Lone Pair Download Scientific Diagram
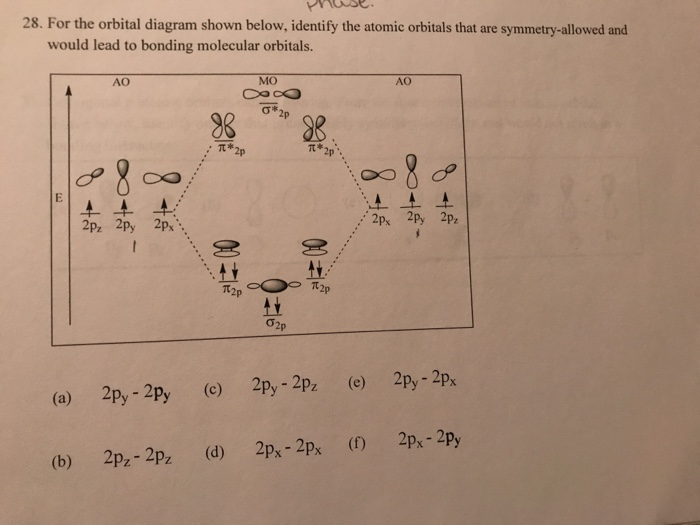
Solved 28 For The Orbital Diagram Shown Below Identify The Chegg Com
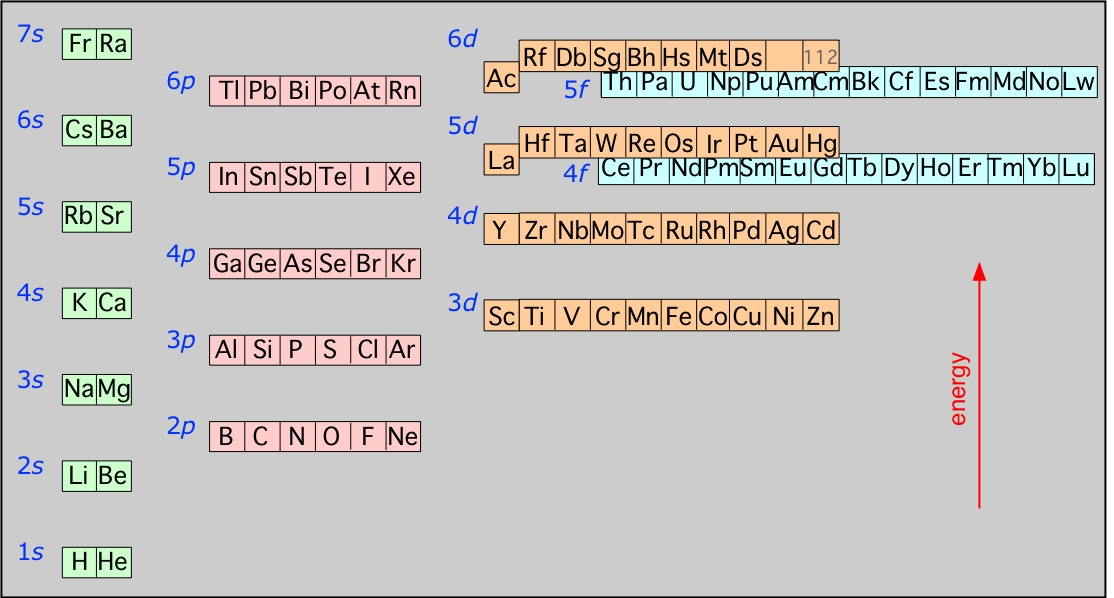
2 7 Atomic Electron Configurations Chemistry Libretexts

6 Order Of Atomic Orbitals Flux Science
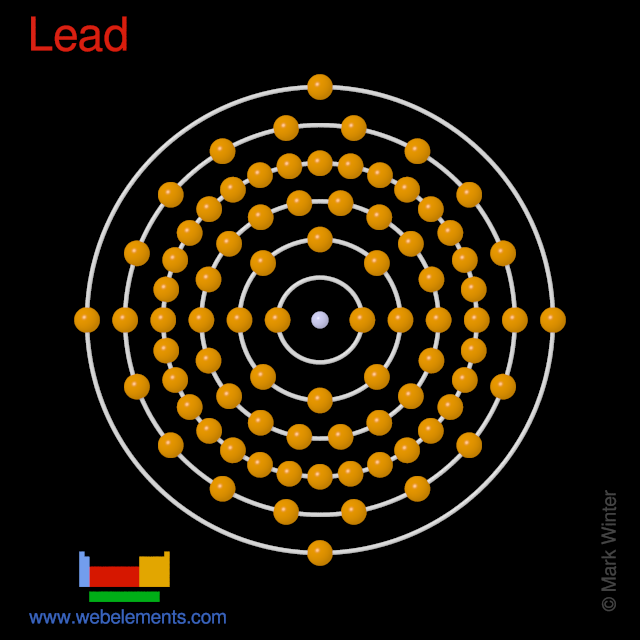
Webelements Periodic Table Lead Properties Of Free Atoms

Molecular Orbital Diagram Wikipedia
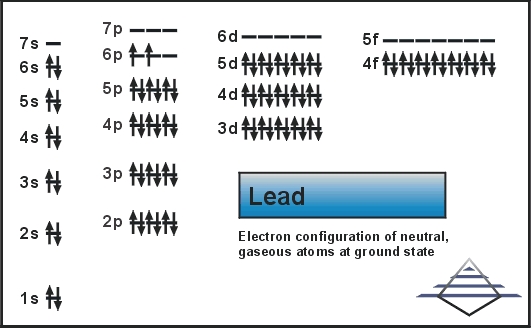
Lead Atom Properties Pilgaard Elements

A Schematic Molecular Orbitals Diagrams Expected For Threecoordinate Download Scientific Diagram

Orbital Filling Diagrams The Cavalcade O Chemistry

Introduction To Molecular Orbital Theory
What Is The Molecular Orbital Diagram For No Quora

Topic 1
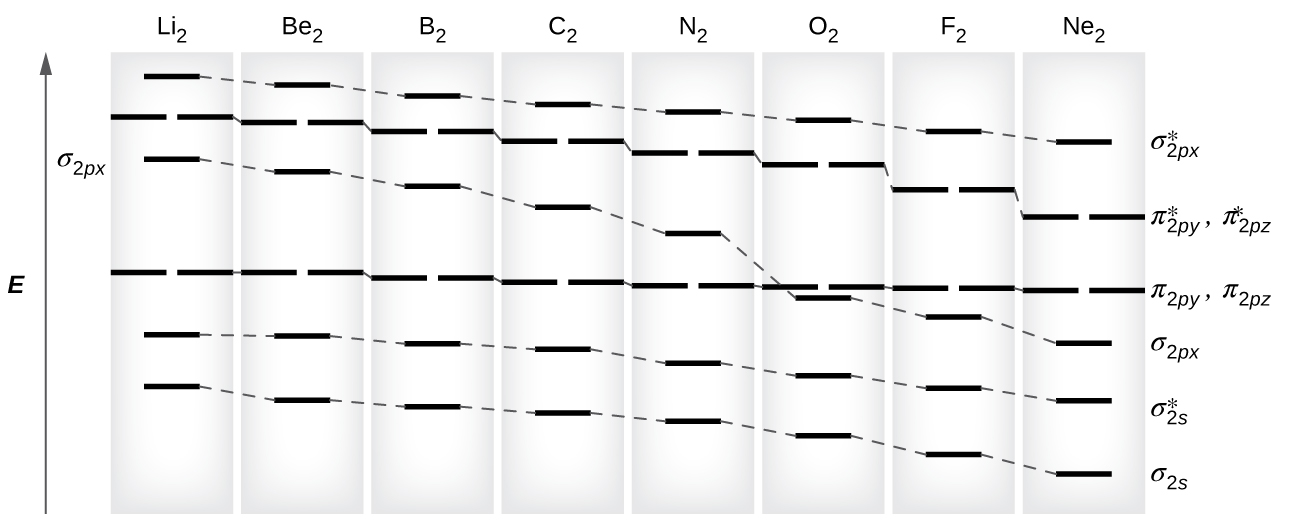
Molecular Orbital Theory Chemistry
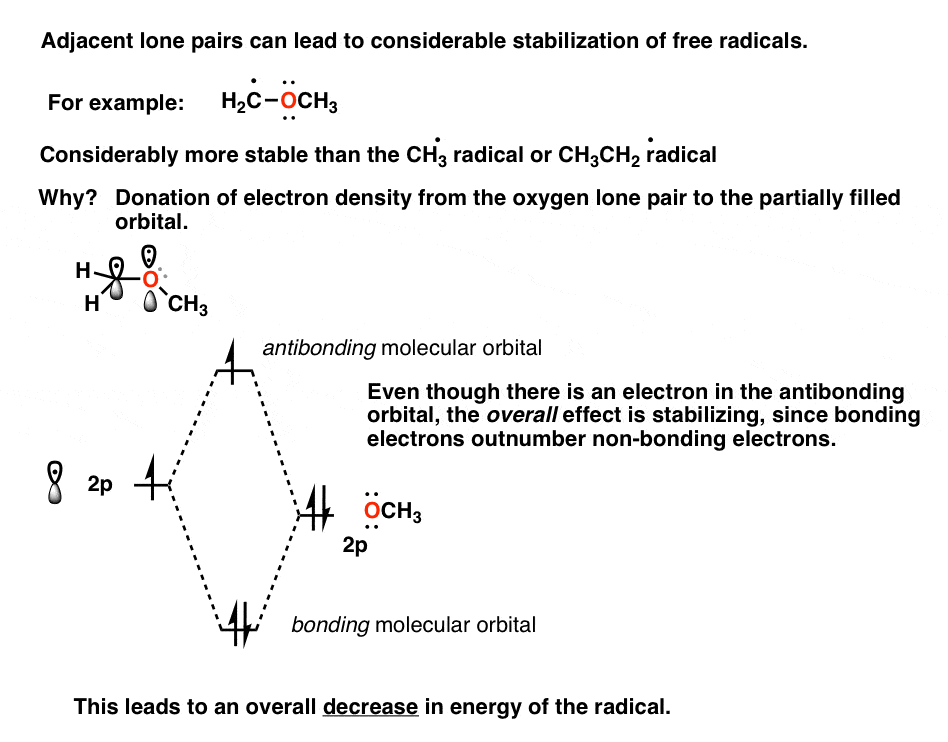
3 Factors That Stabilize Free Radicals Master Organic Chemistry

Construct Molecular Orbital Diagram And Determine Unpaired Electrons In O2 O2 Bn No Homework Study Com
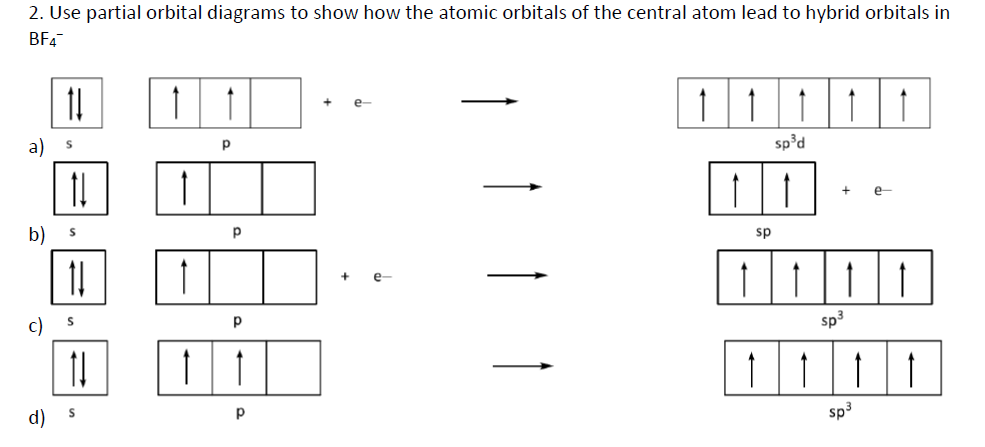
Answered 2 Use Partial Orbital Diagrams To Show Bartleby
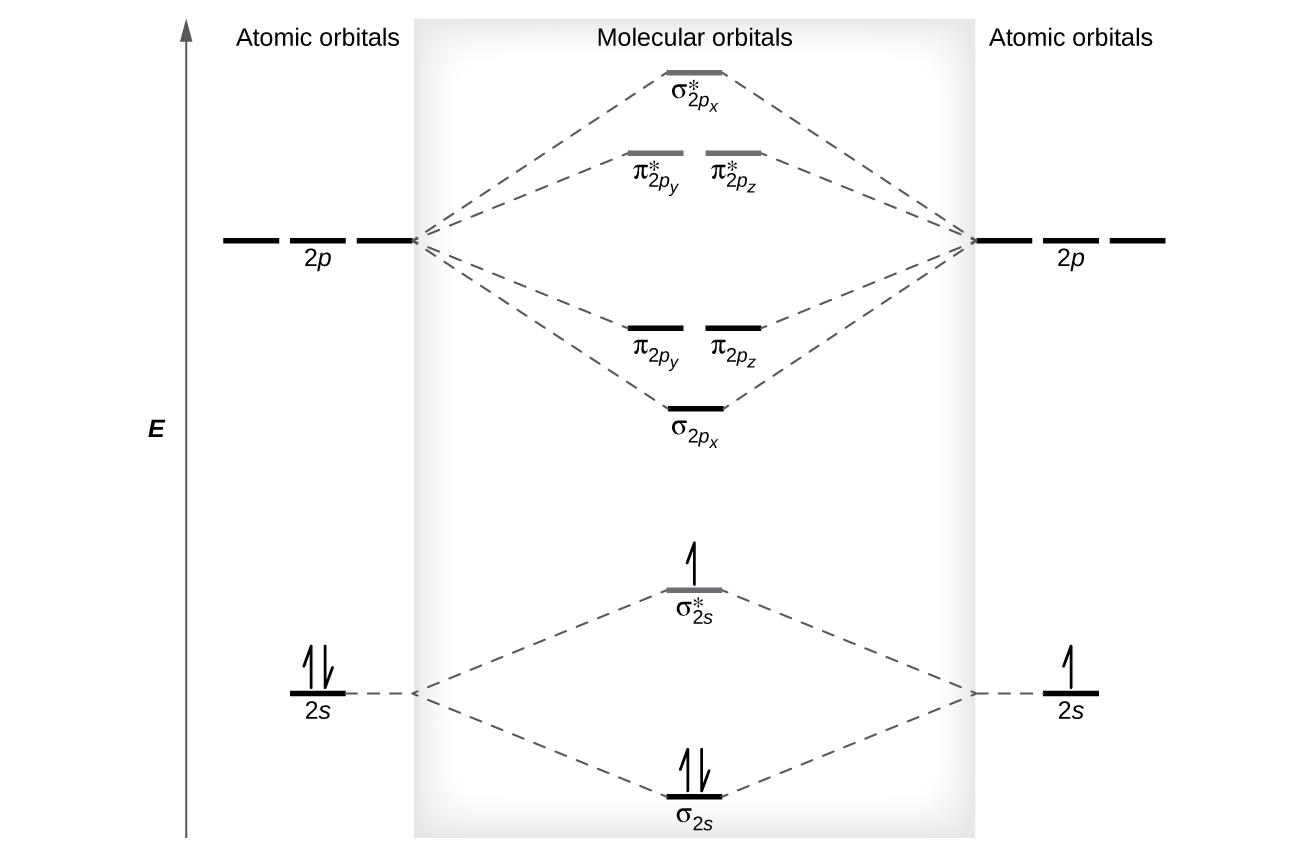
8 4 Molecular Orbital Theory Chemistry Libretexts